العمود الفقري (BMC) نخاع العظام
Mesenchymal Cellular Therapy (Copy)
Spine(BMC) Bone Marrow Mesenchymal Stem Cell
Spine (BMC) Bone Marrow Mesenchymal Cellular Therapy & PRP
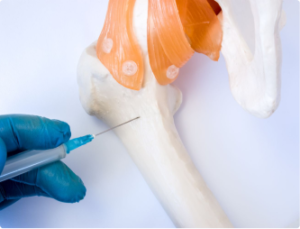
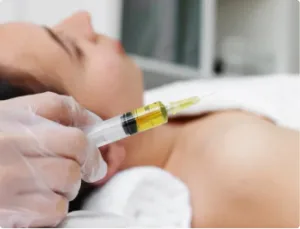
Research in regenerative medicine has shown promising results for treating spinal arthritis and disc issues with mesenchymal bone marrow cellular concentrate and platelet-rich plasma (PRP) therapy. Here are some key findings from medical literature:
Regeneration of Disc Tissue: Studies have demonstrated that mesenchymal cellular matrix derived from bone marrow have the potential to differentiate into cells that can regenerate damaged disc tissue. This process may help repair disc degeneration associated with spinal arthritis and other spinal conditions.
Reduction of Inflammation: Mesenchymal cells have anti-inflammatory properties, which can help reduce inflammation in the spinal discs and surrounding tissues. By modulating the immune response, these cells may alleviate pain and improve symptoms associated with spinal arthritis.
Enhanced Healing: Platelet-rich plasma (PRP) contains growth factors and cytokines that promote tissue healing and regeneration. When combined with mesenchymal stem cells, PRP may enhance the therapeutic effects of cellular therapy, leading to improved outcomes for patients with spinal disc issues.
Clinical Efficacy: Clinical studies have reported positive outcomes for patients undergoing mesenchymal cellular therapy and PRP injections for spinal arthritis and disc issues. These treatments have been associated with reductions in pain, improvements in function, and regenerative changes observed on imaging studies.
While further research is needed to fully understand the long-term efficacy and safety of these treatments, the available evidence suggests that mesenchymal bone marrow cellular concentrate and PRP therapy hold promise as potential interventions for spinal arthritis and disc issues. It’s important for patients to discuss these treatment options with their healthcare providers to determine the most appropriate course of action based on individual needs and medical history.
These statements have not been reviewed by the FDA. Individual results may vary. Literature can be looked up at www.ClinicalTrials.Gov